
Treatment Options
CAR T Cell Therapy
Immunotherapy enhances the power of a patient’s immune system to attack tumors. An immunotherapy approach, called chimeric antigen receptor (CAR) T cell therapy, uses patients’ own immune cells to treat their cancer.
CAR T cell therapy provides engineered molecules called chimeric antigen receptors (CARs) that recognize and destroy antigens present on the surface of lymphoma cells. T cells are removed from patients and genetically modified to produce CARs. The genetically engineered CAR T cells are grown in the laboratory until they number in the billions and are then infused back into the patient.
CAR T Cell Process
1. Leukapheresis
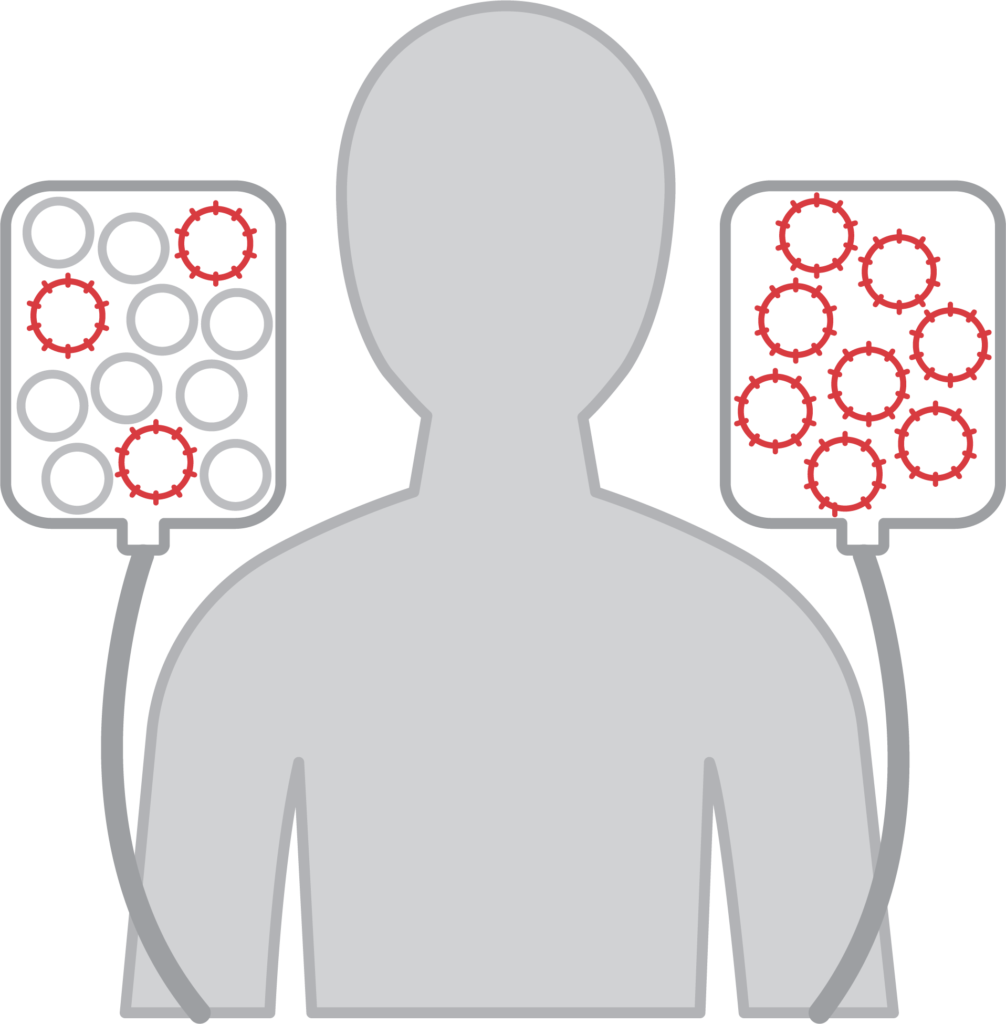
Your T cells are obtained through a process called leukapheresis, which usually takes three to four hours.
2. T-Cell Engineering
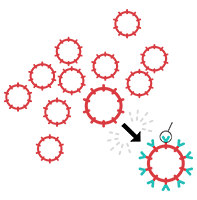
The T cells are sent to a processing center where they are genetically engineered to target your lymphoma.
3. CAR T Cell Transport
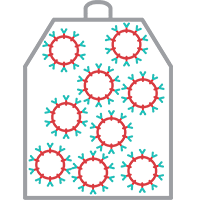
Once enough of the CAR-T cells are available at the processing center, the cells are frozen for transport to your certified treatment center.
4. Lymphodepleting Chemotherapy
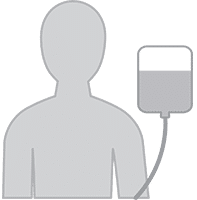
A few days prior to your CAR-T cell infusion, you will receive low-dose chemotherapy.
5. CAR T Cell Infusion
A few days after completing chemotherapy, you will receive your CAR-T cells at your certified treatment center.
6. CAR T Cells Attack the Lymphoma
Once the CAR-T cells enter your body, they begin to multiply and attack the lymphoma cells.
Approved CAR T Cell Therapies in Lymphoma
Approved CAR T Cell therapies include:
- Axicabtagene Ciloleucel (Yescarta)
- Treatment targeting CD19 for patients with certain types of relapsed/refractory large B-cell lymphoma after at least two other kinds of treatment. (DLBCL not otherwise specified; primary mediastinal large B-cell lymphoma; high-grade B-cell lymphoma; DLBCL arising from FL).
- For the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy
- Brexucabtagene Autoleucel (Tecartus)
- Treatment targeting CD19 for patients with relapsed or refractory mantle cell lymphoma (MCL).
- Lisocabtagene Maraleucel (Breyanzi)
- Treatment targeting CD19 for patients with relapsed or refractory CLL/SLL, relapsed or refractory follicular lymphoma, relapsed or refractory mantle cell lymphoma, or certain types of large B-cell lymphoma, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B, who have not responded to, or who have relapsed after, at least two other types of systemic treatment.
- Tisagenlecleucel (Kymriah)
- Treatment targeting CD19 for patients with certain types of relapsed/refractory large B-cell lymphoma after two or more lines of systemic therapy. (DLBCL not otherwise specified; high grade B-cell lymphoma; DLBCL arising from FL)